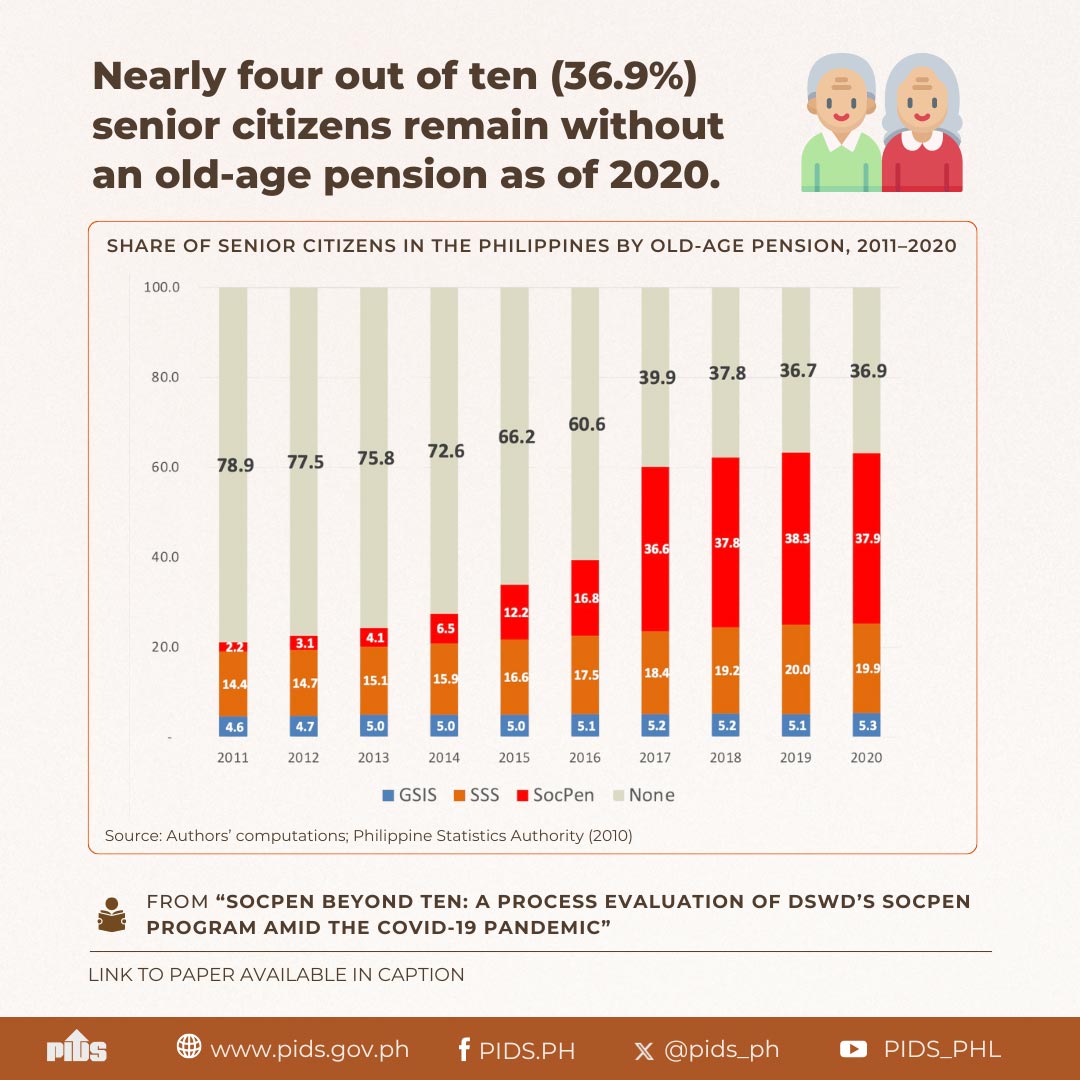
The regulatory process for modern biotechnology applications in the country should be improved.
This was emphasized by Department of Agriculture (DA)’s Bureau of Agriculture and Fisheries Standards Director Vivencio Mamaril, who served as a discussant in a webinar recently organized by the Philippine Institute for Development Studies (PIDS) featuring the study “Modern Biotechnology Application and Regulation in the Philippines: Issues and Prospects”.
According to Mamaril, there have been regulatory inefficiencies under the Joint Department Circular (JDC) 2016-01 that led to delays in finishing the review of applications. Under this JDC, DA was joined by the Department of Science and Technology, Department of Environment and Natural Resources, Department of Health, Department of the Interior and Local Government, and Institutional Biosafety Committees.
“When the JDC 2016-01 was enforced, we had applications reaching 800 calendar days…Shocking as it is, but that is the truth. Kaya yung ‘bureaucratic inefficiencies’ [pointed out by the PIDS study], harsh siya, but guilty as charged,” Mamaril said.
The study noted that while JDC 2016 was supposed to address the lapses of the earlier regulatory framework, “issues continued to emerge, the most prominent of which were bottlenecks during assessments and public consultations.”
The JDC underwent assessment in 2021, and several key changes were proposed. These include replacing the renewals on permit validities with one-time approvals.
“One good thing in JDC 2021 is the nonrenewal. You are only given one certificate, which stays forever unless revoked otherwise,” Mamaril said.
Meanwhile, the BAFS director also raised a concern regarding the business aspect of modern biotechnology crops, including the Bt eggplant—the first application the Bureau received under the new circular.
“My take on the Bt eggplant is if ever it gets approval, what is next? We know that researchers and scientists are good at producing technology, but [it might be a different matter in terms of business feasibility]. It is [interesting] to see if ever it gets approved,” Mamaril pointed out.
In his closing statement, Mamaril concluded that “from a regulator’s point of view, the truth about regulatory delays and bureaucratic processes hurts”.
“Fixing these will be a challenge for us regulators, but we will do our best to address the regulatory delays,” Mamaril added. ###
You may watch the webinar at https://fb.watch/cwSaoRtYFg/ or https://youtu.be/vpvzBhKJBf8.
For more videos of PIDS events, go to https://www.pids.gov.ph/videos.